Two winning stocks in the race to cure Covid-19
Shares in Johnson & Johnson and AIM-listed Novacyt have surged as both firms fight coronavirus.
31st March 2020 12:39
by Graeme Evans from interactive investor
Shares in Johnson & Johnson and AIM-listed Novacyt have surged as both firms fight coronavirus.

For Wall Street giant Johnson & Johnson (NYSE:JNJ) and AIM-listed biotech testing firm Novacyt (LSE:NCYT), the stakes have never been higher as the world awaits a Covid-19 vaccine or full-blown virus testing.
Both companies have this week provided encouraging updates on their progress, with Johnson & Johnson placing itself among the frontrunners in the race to create a widely available and affordable vaccine. It’s just confirmed hopes that its human clinical studies can begin by September, with the first batches potentially ready for emergency authorisation early next year.
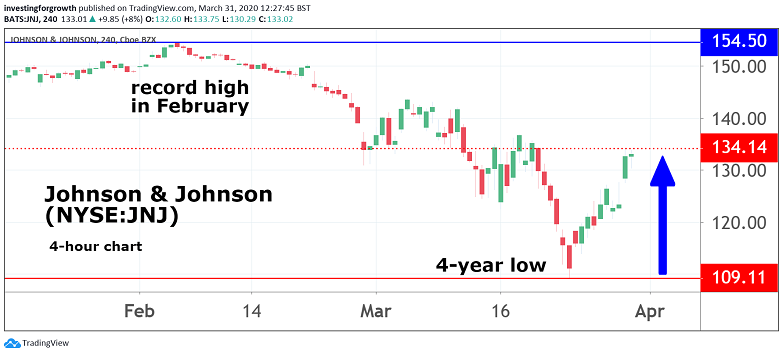
It is hard to argue with chief executive Alex Gorsky when he says that the development represents one of the most pivotal moments in his company's 134-year history. And, in a market shorn of positive headlines, it should at least give investors some cause for optimism.
Source: TradingView Past performance is not a guide to future performance
Johnson & Johnson is not alone in its work, with the Association of Investment Companies reporting that as many as 40 vaccines are in development as scientists carry out research at a speed rarely seen before. Two candidates are already said to be in clinical trials, with at least one of these allowed to skip the animal research stage.
During the swine flu pandemic of 2009, a vaccine was delivered within around six months of the World Health Organization declaring an emergency. The typical vaccine development process takes five to seven years before the candidate is considered for approval.
But even if the Covid-19 trials are successful, there's still the huge challenge of making the vaccine widely available, initially to healthcare workers.
Johnson & Johnson has set aside US$1 billion with its development partner, US Biomedical Advanced Research & Development Association, towards both the research costs and increasing its manufacturing capacity. The healthcare company hopes this will allow it to supply over one billion vaccine doses to the public on a not-for-profit basis for emergency pandemic use.
- Coronavirus cures: This stock is the current front-runner
- Novacyt confirms big demand for its coronavirus test
- Coronavirus cures part two: Can the outsiders race through?
- Take control of your retirement planning with our award-winning, low-cost Self-Invested Personal Pension (SIPP)
The challenge of ramping up production is already an issue for Novacyt as it looks to meet huge global demand for its CE-Mark approved coronavirus test.
Its Southampton-based Primerdesign division has now sold and received orders for over £17.8 million of its CE-Mark and research-use only tests.
The rate of demand continues to increase, with Friday seeing the largest single order to date of £1.4 million from a new customer in India.
Novacyt is now selling its test to more than 80 countries, with the Middle East becoming the strongest selling region. Orders worth £1.6 million have been received from the region in less than two weeks. It is also supplying more than 21 hospitals across the UK.
Source: TradingView Past performance is not a guide to future performance
CEO Graham Mullis said yesterday: “Despite our best efforts to ensure we are fully prepared to meet demand, the rate of sales growth is beginning to be restricted by our ability to scale-up manufacturing as quickly as we would like.
“This, therefore, remains an immediate priority for the company and it is our objective to make as many tests available to as many clinicians around the world as quickly as possible.”
Novacyt hopes it will be in a position to produce four million tests per month, having committed to purchasing additional raw materials for a total of 18 million Covid-19 tests.
It said its Dresden, Germany-based partner BioType was making excellent progress in scaling up production for delivery to Primerdesign's Southampton site for final assembly. The company is also receiving batches of certain products from UK-based Yourgene Health.
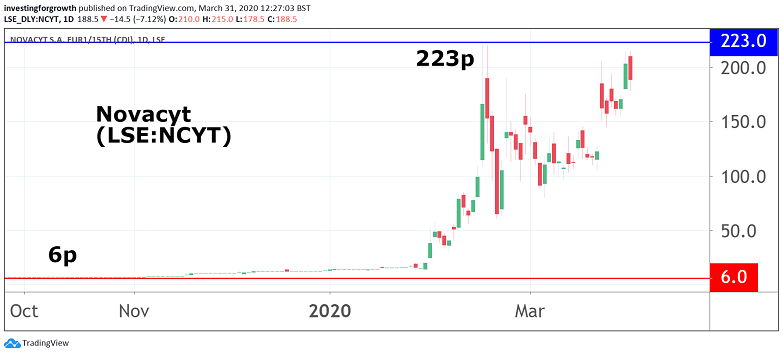
Novacyt's fortunes as an AIM stock have been transformed by the coronavirus outbreak, having previously developed molecular tests for other major incidents, such as the outbreaks of Swine Flu, Ebola in 2014 and Zika in 2016.
The Paris and Camberley-based group listed on the junior market in 2017 at a price of 59.4p in a move that raised £7.1 million. Shares saw a steady descent to as low as 6.1p in October last year, before peaking at almost 223p last month, then closing at 203p yesterday on the back of the latest trading update.
Johnson & Johnson shares, meanwhile, rose 8% last night and were up a further 2% in pre-market dealings today. They had been at a two-year low earlier this month.
These articles are provided for information purposes only. Occasionally, an opinion about whether to buy or sell a specific investment may be provided by third parties. The content is not intended to be a personal recommendation to buy or sell any financial instrument or product, or to adopt any investment strategy as it is not provided based on an assessment of your investing knowledge and experience, your financial situation or your investment objectives. The value of your investments, and the income derived from them, may go down as well as up. You may not get back all the money that you invest. The investments referred to in this article may not be suitable for all investors, and if in doubt, an investor should seek advice from a qualified investment adviser.
Full performance can be found on the company or index summary page on the interactive investor website. Simply click on the company's or index name highlighted in the article.