Novacyt confirms big demand for its coronavirus test
Its test could be quicker and better than the rest, but what will Novacyt make from it?
7th February 2020 14:12
by Lee Wild from interactive investor
Its test could be quicker and better than the rest, but what will Novacyt make from it?
Providing a quick and accurate test for the coronavirus has been the making of Novacyt (LSE:NCYT). It’s currently the second best-performing stock on AIM so far in 2020, returning 265%. Only Baron Oil (LSE:BOIL) has done better. But how do we properly understand its potential?
Few had heard of the company before the coronavirus outbreak in Wuhan, China at the end of December. Now, from their 6p low late last year, Novacyt shares are up a staggering 825%.
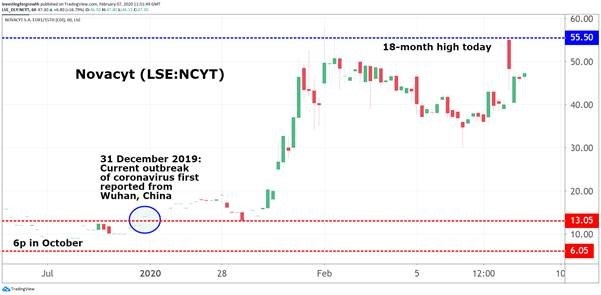
Source: TradingView Past performance is not a guide to future performance
While it is difficult talking about profiteering from human tragedy, it is the job of commercial companies like Novacyt to help with outbreaks like this, and either contribute to finding a cure, or limit the spread of disease.
And Novacyt has been working hard. A new molecular novel coronavirus (2019-nCoV) test was launched at the end of January, just days after we first heard the company’s Primerdesign division was developing one. Its test can give a result in under two hours and, unlike other tests, need not be kept cold during transportation.
Today, Novacyt reports “strong demand” for the ‘research use only’ (RUO) test, with orders for 33,000 tests already received. It is supplying quotes for a further 32,000 from over 30 countries, and notes that many quotes are being converted to orders.
“The directors believe the company is well placed to support the growing global demand for nCoV testing,” it says.
“The company is also pleased to announce it is planning to launch a CE-Mark approved nCoV test in the week commencing 17 February. This approval will mean the nCoV test can be used for clinical diagnostic testing, as well as expanding its use within laboratories. The Directors believe that Primerdesign will be one of the first companies to market its test with CE-Mark approval.”
What’s more, Novacyt has applied to the US Food and Drug Administration (FDA) for Emergency Use Approval (EUA) of its test. Approval would let labs in the US use it for clinical diagnosis on a temporary basis. It is also talking to NHS hospitals and Public Health England, and some tests have already been sold.
- Coronavirus cures: This stock is the current front-runner
- What if AIM share Novacyt helps defeat coronavirus?
While today’s announcement is clearly good news for both human health and Novacyt’s shareholders, greater clarity is needed to identify just how much potential there is for this nCoV test.
Currently, there is lots of guess work involved and ‘back of a fag packet’ calculations, although digging around in the prospectus issued in October 2017, just before Novacyt joined AIM at a share price of 59.38p, does offer a few clues.
Its genesig q16 testing instrument was selling for €5,450 in 2017, and the consumable reagents used with it for anywhere between €10 and €50 per test.
For context, the Primerdesign division increased sales to €6.2 million (£5.5 million) in 2018, with a gross margin of 84%. Novacyt expects the entire business to have generated €13.1 million of revenue in 2019, down 5% on the €13.7 million it posted in 2018, and adjusted EBITDA somewhere below the previous year’s €0.6 million.
Primerdesign was bought by Novacyt for an initial €11 million plus extras in May 2016. It had been set up 11 years earlier by Dr Rob Powell as a spin-out from Southampton University, and had already developed molecular testing kits for outbreaks of Swine Flu in 2009, Ebola in 2014 and Zika in 2016.
Investors will have to wait until April for Novacyt's annual results, although one might expect further updates before then, especially if the virus remains a threat and continues to spread.
These articles are provided for information purposes only. Occasionally, an opinion about whether to buy or sell a specific investment may be provided by third parties. The content is not intended to be a personal recommendation to buy or sell any financial instrument or product, or to adopt any investment strategy as it is not provided based on an assessment of your investing knowledge and experience, your financial situation or your investment objectives. The value of your investments, and the income derived from them, may go down as well as up. You may not get back all the money that you invest. The investments referred to in this article may not be suitable for all investors, and if in doubt, an investor should seek advice from a qualified investment adviser.
Full performance can be found on the company or index summary page on the interactive investor website. Simply click on the company's or index name highlighted in the article.